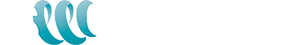Coal Water – Tiny Particles, Huge Problem
5 July 2022
Clean Tech Success
2 August 2022Lithium Extraction - The Mineral We Didn't Know We Needed
Lithium is a highly reactive alkali metal that offers excellent heat and electrical conductivity. However, there are many factors contributing to the lithium industry’s wastewater which is produced during the extraction process. Toxic waste is a major problem in the lithium brine extraction process. There is an abundant reserve and increasing demand, for the extraction and separation of lithium from salt-lake brines worldwide. During lithium extraction from brine, undesirable ions are removed by sequential evaporation/precipitation in large surface ponds, processes. These processes are environmentally intrusive, inefficient, and slow.
What Are The Potential Industries? And Where Is It Applicable?
The world’s demand for lithium brine extraction is growing every day. Lithium has many industrial uses going from glasses, ceramics, pharmaceuticals, and aluminum and magnesium alloys industries. It is used in glass production where lithium increases the durability, corrosion resistance, and thermal resistance for use at extreme temperatures. However, its highest potential use in is the battery industry. It is especially driven by an increased lithium use in the new consumer electronic battery technologies and electric cars in the battery industry. Lithium is used as an electrode and electrolyte material in lithium disposable batteries and lithium-ion rechargeable batteries. Even though many know what lithium is how it is produced remains a mystery to many.
Lithium extraction and processing can heavily depend on the source of the metal. commercial lithium arises from two major sources: brine deposits from underground and mineral ore deposits. Lithium compounds are produced in a variety of forms including lithium carbonate (Li2CO3), lithium oxide (Li2O), and lithium hydroxide (LiOH). The Government of Canada has identified lithium as a critical mineral because it is a key material in the renewable energy transition. Canada shows potential to be a supplier in the renewable energy industry.
How Is Lithium Extracted From The Brine?
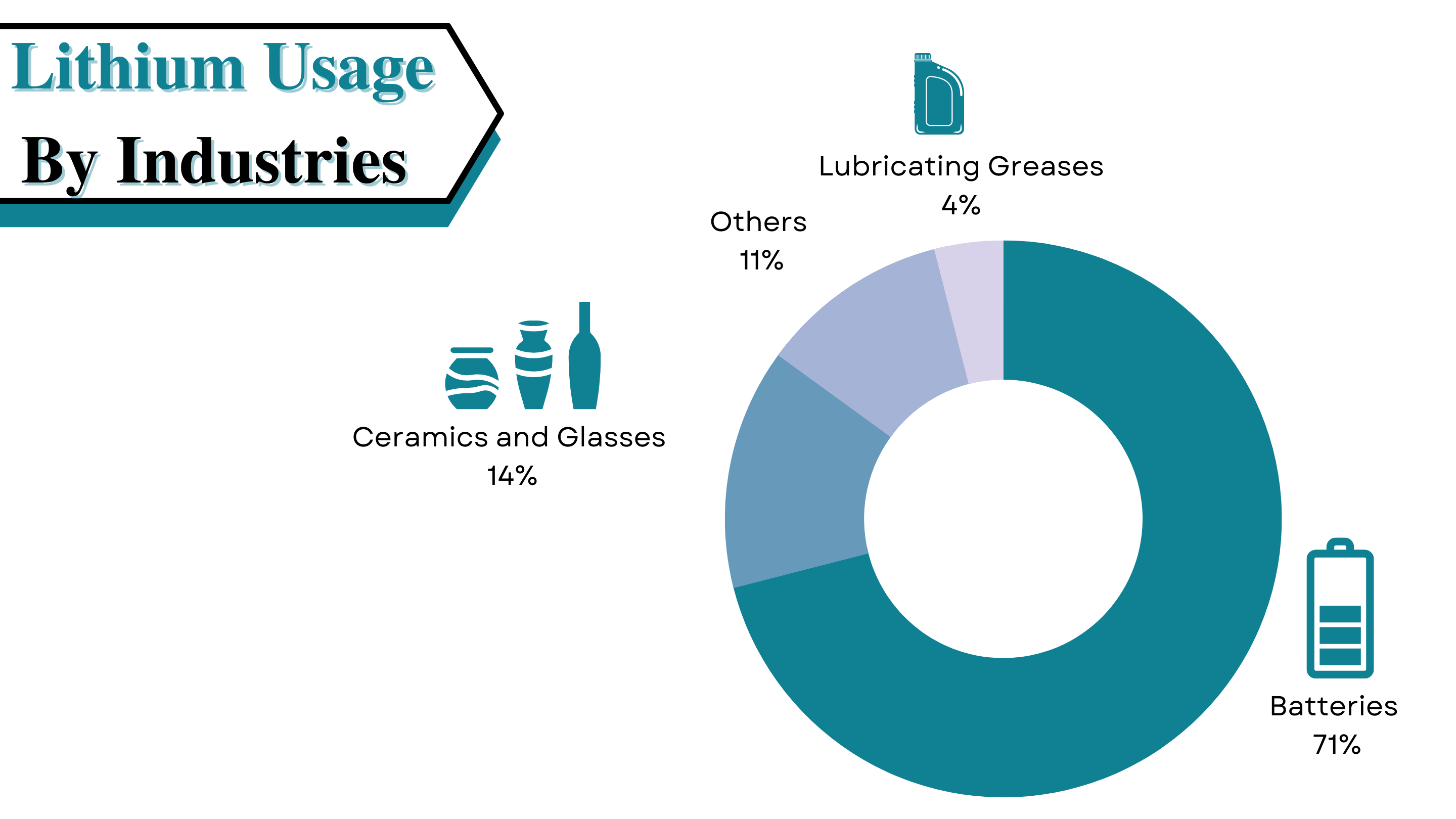
Lithium is the lightest metal on earth, which is so soft that it can be cut with a knife. Extracting this metal isn’t an easy task, the process is done by accessing underground deposits of brine water and ore. Major lithium production takes place in the so-called Lithium Triangle, high up in the Andes, where Bolivia, Argentina, and Chile meet. Only some sources of lithium are cost-efficient and logical for extraction. The extraction done from salt-rich waters first must be pumped to the surface into a series of large evaporation ponds. Where solar evaporation occurs over several months, the extraction process is very straightforward but time-consuming. Over the months, the water slowly evaporates, and a variety of salts precipitate out, leaving brine with high lithium concentration.
At this stage, the brine has significant numbers of unwanted elements. Elements like magnesium hydroxide and calcium boron salts. During the evaporation process, a slurry of the hydrated line (Ca (OH) 2) is added to the brine to get rid of the unwanted elements. When lithium reaches a significant concentration level, the brine is pumped to a recovery facility to extract the metal. This process includes the following steps:
· Brine Purification to remove the left unwanted elements
· Chemical treatment to precipitate out the outcome, i.e., lithium and then filtering it
· Treatment with soda (Na2CO3) to precipitate out lithium carbonate (Li2CO3)
· Washing and drying of lithium carbonate into the final project for its further usage
Another method to extract lithium is mainly from hard rock mines which is done by extracting the mineral from open pit mines and then roasting using fossil fuels. This uncovers dispose of the overburden in the waste dumps and often involves displacing thousands of acres of dirt and rock. The process has major effects on the environment like disrupting nearby land and eradicating plant life.
Why Is Extracting Lithium Important?
The global market for lithium is growing rapidly, the production rose from 25,400 to 85,000 tonnes alone from 2008-2018. Lithium prices have doubled in 2018 because of the increase in demand. The leading cause of an increase in demand for lithium is its use in the batteries of electric vehicles. However, battery usage is not limited to cars there are EVs, laptops, phones, and other digital devices which also use rechargeable lithium-ion batteries. But what is the leading cause for such an increase in demand and production for lithium?
Following are the reasons for this –
· With the increasing demand for electric cars, the need for lithium-ion batteries for its production has obviously gone up
· Rising consumer awareness, in regards to going green and government incentives, is supporting the rise in EVs sales, hence the increase in batteries sale.
· Since 2013, battery prices have fallen by 80%. Also, further cost declines could follow with technological improvements leading to becoming a more feasible option to use.
Most countries have started planning the road map to phase out fossil fuel vehicles, to switch to zero-emission cars. Zero commission cars like battery electric vehicles and fuel cells. It is forecasted that the demand will continue to rise as manufacturers will fulfill the demand for green battery products.

What Are The Problems Associated With Lithium Extraction?
Even though lithium extraction is the new norm, its footprint on the environment cannot be overseen. Green plants and trees remove excess carbon dioxide from the atmosphere during photosynthesis and lithium mining hinders this from occurring. They use heavy machinery which consumes a lot of energy and produces various toxic gases, including carbon dioxide. Locals in some areas complained about increasing droughts, which can threaten livestock farming or vegetation drying out.
The extraction process also led determine to what extent the extraction of saltwater leads to an influx of fresh water and how much it influences the groundwater. The process itself requires a lot of water and to extract one tone of lithium, it takes 500,000 gallons of water approximately. This high-water requirement puts a halt to farming activities in many parts of the world. This can mean mining the metal makes little economic sense but leads to depleting the fertile land. The brine lost 95% of the used gallons of water for the precipitation process to begin. The lithium extraction process lets too much water to evaporate.
Swirltex’s Solution
With the extraction process, there is a significant amount of wastewater generated which becomes impossible to reuse. So, what are we going to do if we run out of water needed to produce lithium? How can we prevent this? Swirltex’s innovative technology can satisfy both economic and environmental concerns. Its unique membrane system can separate solids and wastewater based on buoyancy. Swirltex’s patented technology improves removal efficiency without the costly, long-term operational issues associated with conventional filtration systems. Our system beats the rest because we leave a low environmental impact, have low energy consumption, and provide permeate with high quality.